Philips CPAP Lawsuits








CPAP and BiPAP Sleep Apnea Machines
Tittle and Perlmuter is examining sleep apnea machine cases from users of the Philips CPAP or BiPAP sleep apnea machines. If you or a loved one used a Philips sleep apnea machine for six months or longer and have been diagnosed with a respiratory condition like pulmonary fibrosis or cancer, you might be entitled to financial compensation. Our attorneys can help. For a free consultation, contact our firm or give us a call at 216-222-2222.

See If You Qualify for a Philips CPAP Lawsuit
CPAP Machines
Continuous positive airway pressure (CPAP) therapy uses a machine to help people with obstructive sleep apnea breathe more easily during sleep. A CPAP machine helps increase air pressure in the throat by sending a steady flow of oxygen to the nose and mouth, so that the airway doesn’t collapse when breathing in during sleep. Specifically, a CPAP machine’s compressor (motor) produces a continuous stream of pressurized air that travels through an air filter into a flexible tube. This tube then delivers purified air into a mask that’s sealed around the nose or mouth. During sleep, the CPAP’s airstream pushes against any blockages and opens airways so that the lungs can receive ample oxygen. This helps prevent obstruction of oxygen so that there are not pauses in breathing during sleep. As a result, repeatedly waking up to resume breathing is avoided. CPAP machines are the most commonly prescribed devices for treating sleep apnea disorders.
BiPAP Machines
“BiPAP” is a trade name and BPAP is the device type. Bilevel positive airway pressure (BPAP) machines are used as part of a non-invasive ventilation therapy to ease breathing. They are used in hospitals as well as homes, in which those machines are more compact. Like other ventilators, BPAP machines use pressure to push air into the lungs. They help open lungs which improves oxygen levels in blood and decreases carbon dioxide. BPAP machines are bilevel because they have two different air pressure settings:
- Inspiratory positive airway pressure (IPAP): BPAP machine delivers more air pressure when breathing in
- Expiratory positive airway pressure (EPAP): BPAP machine reduces air pressure when breathing out Some BPAP machines even have a timer that can be programmed to maintain a certain number of breaths per minute. Because they are noninvasive, BPAP machines are a commonly preferable to intubation or used to ensure proper breathing after intubation.
Ventilators
This device is designed to help mechanically control or assist breathing by delivering a certain percentage of oxygen in the breathing gas.
Issues with Philips CPAP and Sleep Apnea Machines
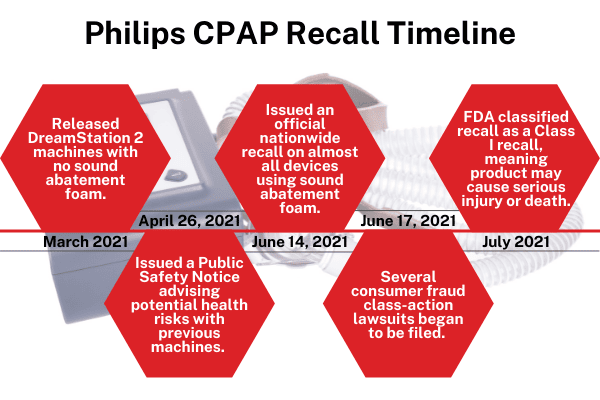
Philips recalled over 3 million of their CPAP, BiPAP, and ventilator devices in June of 2021 due to potential health risks. The sound abatement foam used to keep the machines quiet and reduce vibration may degrade and release particles and toxic gases into the airways that users inhale or swallow. Lab tests revealed degraded foam and gases several toxic and cancer-causing chemicals. Devices that are more than three years old or exposed to high heat or humid environments are reported to be more likely to have degraded foam. Inhaling or ingesting foam particles or gases may cause toxic, carcinogenic, and respiratory effects.
In July 2021, the FDA classified the Philips CPAP recall as a Class I recall which means the product may cause serious injury or death.
Philips CPAP Lawsuits Are Based On 2 Violations
- Design Defect
The devices that Philips sold to patients were defective and dangerous. The foam they used in devices is so dangerous that it can cause cancer when it degrades. - Failure to Warn
Philips did not warn the public as soon as they became aware of the risks. There are allegations that users have been complaining for years about health issues due to the foam.
Philips Negligence
Since the recall, several consumer lawsuits have been filed across the country. The complaints contain specific factual allegations stating that Philips had been receiving complaints for years from CPAP users who reported that they were inhaling foam or dust-like particles from their device.
Philips also disclosed problems with the recalled devices to investors before issuing the recall. They announced the nationwide safety recall on June 14th, 2021; however, several weeks prior, Philips told its corporate investors about the problems with the DreamStation and other CPAP devices. On April 21st, 2021, Philips released its quarterly earnings report to investors that addressed problems and health risks related to the DreamStation line of devices in the “Regulatory Update” section of their report.
The company just so happened to release a new and safer alternative, DreamStation 2, just weeks prior to the recall. This new device was designed and manufactured without using sound abatement foam. A few weeks after the new launch, Philips notifies the public that the older DreamStation devices are not safe and then shortly after issues a recall. This shows a clear effort to time the recall to minimize financial losses. Products like these take months and sometimes even years to design and manufacture. This makes it obvious that Philips has been aware of the issues for longer than they’ve led the public to believe.
Case Criteria for a Philips CPAP Lawsuit
Qualifying criteria for filing a CPAP lawsuit include:
- You used one of the recalled CPAP, BiPAP, or other Philips sleep apnea machines every night for six months or longer
- After using the recalled CPAP device for at least six months, you suffered from any of the following health conditions:
- Pulmonary fibrosis, any injury involving your respiratory system, or diagnosis with a respiratory condition
- Damage to your liver or kidney
- Diagnosis with lung cancer, kidney cancer, liver cancer, or any type of cancer
Philips CPAP Machine Lawsuit Settlement
If we can determine that you have a lawsuit, we believe you are entitled to compensation. If you file a lawsuit and our hardworking attorneys are successful in negotiating your case or we win at trial, you could be compensated for the following:
- Medical Bills – You could be entitled to payment for past medical bills as well as any future medical treatment or procedures you might need that are related to your use of a defective Philips device.
- Lost Income – You could recover income you lost while in the hospital because of a defective Philips device. This could also include income you might lose in the future if you are no longer able to work.
- Pain and Suffering – Patients place a great deal of trust in medical devices. It’s assumed that they are safe and that the manufacturers aren’t knowingly withholding information about their safety. When you have been severely injured or impacted by their negligence, it can be emotionally painful or traumatizing. You could be entitled to compensation for this pain or suffering.
- Punitive Damages – Punitive damages are awarded to punish companies and deter them from practicing negligent behavior in the future. Philips continued to sell their devices even though they were aware of their defects. This is unethical and dishonorable. Due to the severity of your case, a court could award punitive damages.
What To Do If Your CPAP Device Has Been Recalled
The FDA recommends that people using a recalled Philips CPAP machine should:
BiPAP or CPAP:
- Talk to their medical provider to discuss treatment options which could include:
- Stop your CPAP treatment
- Research another CPAP machine that is safe
- Try other treatments like oral appliances or positional therapy
- Initiate long-term therapies like losing weight, quitting smoking, limiting alcohol, or surgical options
- Continue using your affected device if the benefits outweigh the risks identified in the recall notification
- Follow the manufacturer’s instructions and recommended cleaning and replacement guidelines.
- Register your device on the Philips Respironics recall website
- Report any problems with a device through the FDA’s MedWatch Voluntary Reporting Form
Ventilators:
- Do not stop or change ventilator use until talking with your health care provider
- Talk to your health care provider about using an inline bacterial filter, which could help filter out particles of foam.
- Register your device on the Philips Respironics recall website
- Report any problems with a device through the FDA’s MedWatch Voluntary Reporting Form
Tittle & Perlmuter Can Help
At Tittle & Perlmuter, our job is to fight for you. We will help you to the best of our ability. We will be on your side and our goals will be the same as yours. Our Cleveland personal injury lawyers take a comprehensive approach to helping victims of negligence. If you or a loved one has suffered any health consequences from using these Philips medical devices, contact our firm for a free consultation or give us a call at 216-285-9991. We can help determine if you are eligible to file a lawsuit.
Frequently Asked Questions
Sleep apnea causes interruptions or pauses in breathing, often because the throat or airways briefly collapse, or something temporarily blocks them.
Philips recalled device brands include:
- Trilogy 100 Ventilator
- Trilogy 200 Ventilator
- Garbin Plus, Aeris, LifeVent Ventilator
- A-Series BiPAP Hybrid A30
- A-Series BiPAP V30 Auto Ventilator
- A-Series BiPAP A40
- A-Series BiPAP A30
- E30
- DreamStation ASV
- DreamStation ST, AVAPS
- SystemOne ASV4
- C Series S/T, AVAPS
- OmniLab Advanced Plus
- System One 50 series
- System One 60 series
- DreamStation CPAP, Auto CPAP, BiPAP
- DreamStation GO CPAP, APAP, Auto CPAP
- Dorma 400, 500 CPAP, Auto CPAP
Most of the recalled devices are from Philip’s first-generation DreamStation line. The recall affects all serial numbers of the listed devices manufactured between 2009 and April 26,2021. Philips reported that more than half of the affected devices are in the United States.
In addition to any general Philips CPAP side effects, recalled machines have other unique risks because of exposure to particles or gases from degraded foam. These risks include chemical exposure risk, toxic effects, cancer, and respiratory issues. Bladder cancer, lung cancer, and stomach cancer have been linked to CPAP.
Potential risks associated with recalled devices:
- Irritation (skin, eye, and respiratory tract)
- Cough
- Chest pressure
- Sinus infection
- Inflammatory response
- Headache
- Asthma
- Organ problems (kidneys and liver)
- Carcinogenic effects (risk of various cancers)
- Hypersensitivity
- Nausea/vomiting
Philips recalled several of their BiPAP and CPAP devices because they contain a sound abatement foam that degrades. Particles from the foam can be inhaled or ingested and cause cancer or serious respiratory issues.
The toxic foam is polyester-based polyurethane. Because the foam degrades into small particles than can be inhaled or ingested, it is causing people severe health issues. The foam also produces a toxic gas which can lead to cancer or other medical issues.
These damaging devices manufactured by Philips have been sold to patients since 2009, which means that some users have been exposed to the dangerous foam and toxic gases for more than a decade.
You may be eligible to file a lawsuit if you used any of the Philips devices they recalled. Some details that can help your case are knowing which device you used, when you used it, and how long you used it for. Our firm will need to know if you have been diagnosed with cancer or any other health condition that you and your doctor believe to be related to the recalled device. If you have, you could have a legitimate legal claim against Philips. Our firm can help you obtain medical records and any other additional documentation that is needed while guiding you through next steps.
Types of Qualifying CPAP Machines:
- SystemOne Q Series
- DreamStation CPAP, Auto CPAP, BiPAP
- DreamStation GO CPAP and APAP
- Dorma 400/500 CPAP
- REMStar SE Auto CPAP
- DreamStation ASV
- DreamStation SV/AVAPS
- SystemOne ASV4
- C Series ASV, S/T, AVAPS
- OmniLab Advanced Plus In-Lab Titration Device
- E30 Continuous Ventilator
- Trilogy 100 Ventilator
- Trilogy 200 Ventilator
- Garbin Plus, Aeris, LifeVent Ventilator
- A-Series BiPAP Hybrid A30 (not marketed in the U.S.)
- A-Series BiPAP V30 Auto
- A-Series BiPAP V30 (not marketed in the U.S.)
Contact Our CPAP Machine Lawyers Today
Our attorneys are reviewing potential cases of victims who have been impacted by Philips’ recalled devices. If you meet the qualifying case criteria described above, contact our firm for a free consultation. We are here to fight on your behalf, get the compensation you deserve, and help you navigate through the whole process.

